The figures presented here were selected to illustrate aspects of parenchyma cells and tissues


Paradermal section through the leaf of ground ivy (Glecoma, in the mint family, not a real ivy). The long strands with helical red bands are xylem cells that conduct water. At this point, focus on the larger, rounded parenchyma cells that touch the conducting cells (arrows indicate three of many). The conducting cells are slightly scalloped because the parenchyma cells pressed so firmly against them while they were maturing. The dark material in all the parenchyma cells are chloroplasts packed so closely that it is difficult to tell that they are individual bean-shaped organelles. In parenchyma cells that contact xylem conducting cells, chloroplasts are located along the walls away from the conducting cell. This leaf – like most material used for general studies of plant anatomy – was prepared by embedding it in wax and then cutting it into sections about 10 to 12mm thick. This is too thick to be able to see labyrinthine walls, but almost certainly, the parenchyma cell walls that touch the xylem conducting cells are transfer walls with labyrinthine walls. To be certain, it is necessary to use either scanning or transmission electron microscopy. If these cells do indeed have labyrinthine walls, then they are transfer cells.

. Section of bean cotyledon. These are storage parenchyma cells in a germinating bean seed, storing both starch grains (stained pink) and protein (colorless round or angular particles). The very dark red bodies are nucleoli, and in several cells, the nuclei are visible as a gray mass surrounding the nucleoli. These storage cells remain alive after they have filled themselves, and they will be active in unloading the stored carbohydrates and proteins as the seed germinates. In many monocots like corn, wheat and rice, the storage parenchyma cells die after they have filled themselves, and all unloading is carried out by enzymes secreted by other cells. Many of the walls are indistinct in this section, having taken up only a little of the stain, but where the walls are distinguishable, they are thin.


Transverse section through an acorn (a seed of oak, Quercus). An easy way to identify starch grains is to illuminate them with polarized light. is illuminated with ordinary brightfield (not polarized) light and the starch grains have been stained a pink color. This is typical of starch, but other storage products might also stain like this, so we could not be absolutely positive it is starch based just on this. here is the same area illuminated with polarized light, and the crystalline nature of the starch grains is revealed by their being white with a dark cross running through each one. Notice that the walls – these are primary walls because this is parenchyma – do not shine; if these were secondary walls, they would shine as brightly as the starch grains

Transverse section through a leaf of ice plant (Mesembryanthemumcrystallinum). The large circle protruding from the surface of the leaf is an epidermis cell that stores water. It has a volume hundreds of times larger than that of other parenchyma cells in the leaf, so its water storage capacity is comparatively great, but it also has a large surface area through which water can be lost to the air. The nucleus-to-cytoplasm ratio in this cell is extremely low, but water storage is a simple metabolism and the cell probably does not need a great deal of nuclear control.

Root cells in Clusia. The red-stained particles here are tannins, which are an extremely common storage product for many plants. Storage products such as starch, protein or water are stored temporarily and then are mobilized and used by the plant for its further growth and development. Tannins, in contrast, deter animals from eating the plant material – tannins react with the proteins in the animals’ digestive system, denaturing (tanning) them and thus either killing the animal or encouraging it try eating some other plant. In some plant species, tannins are present as a uniform, smooth substance rather than the particles seen here. As the samples were dehydrated with alcohol, the protoplasts shrank slightly, leaving a white space between the tannin particles and the wall. The space is thus an artifact, but it gives us a good view of the thin primary wall. Small intercellular spaces are present between cells, but no organelles can be distinguished among the tannin particles – these cells might in fact have died even before the sample was collected. Tannic acid, used to tan leather, is extracted from cells like this in insect galls on oaks.

Transverse section of pith in ragweed stem (Ambrosia). The large irregular objects that appear empty are parenchyma cells, the small areas are intercellular spaces (arrows), and the dark lines that make up the image are primary cell walls. Parenchyma cells in pith are often some of the largest cells in any plant, being so large that during the cutting of the section, most of every cell is cut away: These walls appear to be clean and distinct because they come straight up at us -- both the back and front walls of each cell have been cut off. These cells appear empty because each consists almost entirely of central vacuole, and during preparation for microscopy, the vacuole contents leaked out and were washed away -- at this point, the cells really are virtually empty. Because each central vacuole is so large, its vacuole membrane is pushed close to the cell's plasma membrane, which itself is pressed against the wall. There is only a very small amount of cytoplasm, squeezed into such a thin, flat layer near the wall that it is almost impossible to see, even at high power. Several cells here have a faint bluish material in them, that is the protoplasm in face view: the section has just grazed either the front or back wall and the thin layer of cytoplasm. Being so thin, it has absorbed only a little stain, so it appears faint, with a few pink dots that are probably plastids. Nuclei would be large enough to be visible at this magnification, but none is present just due to luck -- in each of the cells here, the nucleus must have been either in front of or behind the cuts that made this section, so the nuclei were cut away.

Transverse section of Aristolochia stem. The parenchyma cell in the very center (arrow) appears to be filled with a weblike mesh, but in fact we are looking at either the front or the back wall, and virtually the entire wall is a set of primary pit fields. The whitish areas that appear to be holes are just areas where the primary wall is particularly thin and filled with plasmodesmata (we cannot see plasmodesmata with ordinary brightfield light microscopy, but these thin areas typically contain high concentrations of plasmodesmata). The darker material that forms the mesh is just primary wall of ordinary thickness. If the microscope illuminator were turned bright enough, it too would appear whitish.

Longitudinal section of stem of milkweed (Asclepias). This micrograph shows three columns of parenchyma cells; those on the left and right have been cut down the center, so their front and back walls are missing, But the middle row of cells has been cut just where those cells are pressed up against and contact another row of cells close to us. The vertically elongate ovals, outlined in material stained brown, are the contact faces: the point where two cells press against each other is the contact face. If both cells are spheres, the contact face is round, but if the two cells are columnar, the contact face is oval, as are these in the micrograph. If the cells barely touch each other, the contact faces are small, but if the two cells are forced firmly against each other, the contact face is larger. Within each contact face are numerous smaller oval areas (arrows), so pale they appear white. They look like holes in the wall but are actually primary pit fields -- areas where the two primary walls of the contact face are unusually thin and plasmodesmata occur in high density (the plasmodesmata cannot be seen by ordinary light microscopy).

Transverse section of stem pith in beet (Beta vulgaris). This is a high magnification view in which part of the front wall of a cell is present, it is the sheet of bluish material. On the upper left, the microtome knife passed through the wall and cut part of it away, leaving just a view of the vacuole (white area). The remaining sheet of front wall is so delicate that it has folded and wrinkled slightly, probably during the agitation that occurs as the slide was being stained. This wall is remarkably smooth and uniform; it has no primary pit fields and although there must be cytoplasm lying against this wall, pink-stained plastids are visible. There is a triangular intercellular space surrounded by bluish material which is the walls of the three cells that meet at the space

Transverse section of rhizome of blue cohosh (Caulophyllum thalictroides in the barberry family) These parenchyma cells are somewhat unusual in that many of them in the section have nuclei. Most living parenchyma cells do have nuclei, of course, but usually the cells are so large compared to the small size of the nuclei that a thin slice of tissue rarely catches many nuclei – the nuclei of most cells are cut away so the cells appear to be nonnucleate. The large number of nuclei here is due to the cells being somewhat small for parenchyma cells, and the section is rather thick (the section is so thick that the nucleus in the upper right hand corner is out of the plane of focus – in a very thin section, it would have been cut away). These cells have abundant gray contents but are not as full as they appear – again, because the section is thick, we are seeing some contents along the front or back wall in most cells. Each cell does have a large central vacuole, but because the vacuoles are colorless, they do not stand out visibly compared to the gray cytoplasm that lies either in front of or behind them.

Transverse section of stem pith of sunflower (Helianthus). This parenchyma tissue is unusual in that all the component parenchyma cells (remember that parenchyma is both a type of cell and a type of tissue) abut each other with no visible intercellular spaces. Parenchyma tissue varies from having only tiny intercellular spaces as here, or much larger ones. Note how straight the cell walls are: all cells are equally turgid – they must all have the same vacuolar pressure pushing the walls against each other. If one cell was more pressurized than its neighbors, its walls would push theirs back – it would be more rounded and the neighbors’ walls would be concave.

Transverse section of leaf of dracaena (Dracaena). This section contains bothsclerenchyma (the two masses of cells with red-stained walls, but not the epidermis along the top) and parenchyma (all the other cells). This figure shows the difference between the thin primary walls of parenchyma cells and the thick secondary walls of the fibers. The primary walls of the parenchyma cells do not contain lignin, so they have not taken up the red stain, but both the primary and the secondary walls of the fiber cells are lignified and have been stained so intensely that the primary walls of the fibers cannot be distinguished from the secondary walls.

Transverse section of the funiculus (the stalk that attaches a seed to a fruit as it develops) in bean (Phaseolus vulgaris). These parenchyma cells have contents that have been stained red, making theintercellular spaces quite visible. The cytoplasmic staining is so intense it is difficult to see nuclei, but in many cells the very dark red, small dot is a nucleolus (arrows), and the nucleus can be detected in some of the cells as a mass with slightly different color surrounding the nucleolus.

Transverse section of leaf of common barberry (Berberis vulgaris). All cells in this micrograph are parenchyma cells. The topmost and bottommost layers are the upper and lower epidermises of the leaf, and all cells in between are technically known as mesophyll (Chapter 12: Leaf). These mesophyll cells have very obvious contents, most of which have been stained red, which is very common in slides of plant tissue (the stain Safranin O stains many different organelles, turning them all red). Cells of the lower epidermis appear rather empty, which makes it easy to identify the nucleus in four cells -- the nuclei are round and are a uniform red. The cells just below the upper epidermis are the palisade parenchyma: each cell is columnar and most have a large red-stained nucleus near the middle of the cell. Almost all of the rest of the red-stained material in those cells consists of chloroplasts. The chloroplasts were green when the leaf was alive, but the alcohol used to preserve the leaf tissues dissolves chlorophyll, bleaching the chloroplasts which then become stained red. Between the palisade parenchyma cells and the lower epidermis are spongy mesophyll cells. Their chloroplasts are so abundant they make it difficult to see the nuclei; on a microscope, the nuclei can be identified by focusing up and down with the fine focus. The primary walls have been stained blue-green, and all are very thin. Intercellular spaces are large in spongy mesophyll but smaller in palisade parenchyma (some of the large white areas in the palisade are regions where the cells were cut away by the microtome knife, and although they appear large, they are very thin).

Transverse section of leaf of magnolia (Magnolia). Palisade parenchyma at a higher magnification than that of Fig. 3.2-1. Thin primary walls are easy to see as fine blue lines; the protoplasts shrank somewhat when the samples were treated with alcohol, so there is a bit of empty, white space between the wall and protoplast in some cells. This artifact makes it easier to see the wall. Several nuclei are present, one is round, others are oval or flattened; all have been stained gray and each has one or two small, dark red nucleoli. Chloroplasts form a single uniform layer, packed together so closely that they cover almost all the wall. In the cell marked X, the front wall is present in the section (we can tell it is the front wall, not the back wall, because the chloroplasts that lie against it are partially obscuring the nucleus; if it were the back wall, the nucleus would hide the chloroplasts). In this cell, you can see that chloroplasts are round in face view. In other cells, side walls come directly up at us, and chloroplasts are visible in side view; in most areas, it is easy to see each one individually. In the areas where they are not so distinct, it is because they are crowded together.
Organelles such as mitochondria, endoplasmic reticulum and dictyosomes are also present, but are never visible by ordinary light microscopy.
Notice that the epidermal cells have thick walls stained a very dark red. Most of that is due to the water-proofing chemical cutin in the walls, but the main point to notice now is that the primary walls of palisade parenchyma cells are extremely thin compared to those of epidermis cells. The layer of rather empty-looking cells between epidermis and palisade parenchyma is called hypodermis.
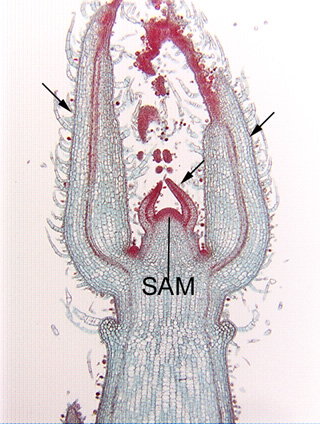
Longitudinal section of the shoot tip of coleus (Coleus). This shoot tip will provide help you understand the orientation of Fig. 3.2-4, which is a close-up of the shoot apical meristem present here at the center of the image (the mass of very dark cells, labeled SAM)). There are two small leaf primordia, one on either side of the apical meristem, and to the outside of them are two much larger leaf primordia that are attached lower on the stem (three of the leaf primordia are indicated by arrows).

Longitudinal section of the shoot apical meristem of Coleus. This is a magnification of the apical meristem shown in Fig. 3.2-3. All cells here are parenchyma cells involved in the synthesis of new cells. Each cell is almost filled by a prominent round, red-stained nucleus (in some nuclei you can see a dark red, dot-like nucleolus). These cells, like most apical meristem cells, are small, not much larger than the nucleus. All organelles are present, but too small to be seen: plastids are present as small proplastids not large chloroplasts, vacuoles are small and scattered rather than being coalesced into a large central vacuole, and all other organelles are never visible by ordinary light microscopy. Because these meristematic cells are so small, cell division -- cytokinesis -- can occur quickly because the phragmoplast and cell plate do not have to grow to a large size before they meet the side walls.

Transverse section of stem of angelica (Angelica, an ornamental herb in the parsley family) The large red-stained cells at the bottom of the micrograph are xylem cells, the larger ones at the top are phloem cells. The band of flat, cuboidal or box-like cells in between xylem and phloem are vascular cambium cells, another type of synthetic parenchyma. Although these appear to be small cells, like those of the shoot apical meristem in Fig. 3.2-4, the fact that so few nuclei are visible indicates something must be not quite as it appears. These are actually extremely long cells that have been cut in transverse section – most cells are probably about 100 to 200mm long and this section is only about 10mm thick, so there is a 10 in 100 to 200 (1 in 10 to 20) chance of cutting through one of these cells in an area where a nucleus happens to be.

Transverse section of wormwood (Artemisia). This circle of dark red cells represents a transverse section of a long, tube-like secretory duct: the entire tube consists of these densely cytoplasmic cells that secrete their product into the duct lumen, the white "empty" space (empty only because the secretory product was washed out as the tissue was processed). The red material in each cell is all the cell's protoplasm, not just its nucleus; even though the red material does look like a lumpy nucleus, it is just too abundant and too lumpy to actually be a nucleus. The red material is also quite abundant just for protoplasm, but secretory parenchyma cells are often densely cytoplasmic with little vacuolation. Only a single layer appears to be secretory, as all surrounding parenchyma cells have protoplasm that has stained less intensely (they are also extremely plasmolyzed -- the fixative probably had too much alcohol in it so it absorbed water from the cells). Cells on the top of the micrograph have thicker walls -- these are collenchyma cells

Transverse section of pine wood (Pinus). This is one of thesecretory ducts in pine that produce the pitch that you may have seen oozing from a wound in a living tree or dripping from a piece of pine lumber. Although these are secretory parenchyma cells, they do not have dense protoplasm like that of ducts in Artemisia These parenchyma cells are rather large and pillowy, and the walls that faces the duct lumen are not smooth and taut but instead are undulate, so they do not show a clean profile. The outer walls of each cell are somewhat thicker than is typical of parenchyma cells

Transverse section of root of Clusia (a tree from tropical Central America with no common name in English). The secretory product of this duct was preserved by the fixation process and has taken up a blue stain (secretory products are often dissolved during processing, so ducts typically appear empty on microscope slides). This duct is lined by one layer of secretory parenchyma cells that appear to be completely empty. Many of the surrounding cells have deposits of tannins that have stained as red particles or as solid red masses. In some, the tannins occur only along the wall, so at first glance the tannin might appear to be a red-stained secondary wall, but a secondary wall would not be as rough or irregular as these tannin deposits. The cell at xxx has a bit of front (or back) wall present, showing the primary pit fields as small whitish dots and splotches.


Transverse section of root of buttercup (Ranunculus). The circular structure in the center of the large micrograph contains the conducting tissues of the root, but now we are concerned only with the outer tissue, the root cortex. All these cortex cells are parenchyma, and because the bulk of the root consists of this (see the inset), this is structural parenchyma. It is also storage parenchyma because most cells are full of starch grains (stained purple). The starch grains (amyloplasts) developed from proplastids and are so abundant that nuclei are hidden. Some cells have few or no starch grains, probably because some grains fell out while the slide was being stained, after the cells had been cut open during microtoming. Because the starch grains are so abundant, it is easy to tell which areas are cells and which are intercellular spaces, and this tissue has a large percentage of its volume as intercellular space. Many botanists would consider the intercellular spaces here sufficiently abundant to call this aerenchyma, others would consider it a bit too compact -- there is no universal definition of aerenchyma.

Transverse section of leaf of ivy (Hedera). Like leaves of most species, this ivy leaf consists of almost pure parenchyma, except for its veins. Even though ivy leaves are somewhat tough and leathery, their upper and lower epidermis and all the photosynthetic tissues are composed of parenchyma cells. We can consider the parenchyma to bestructural because it makes up most of the bulk of the leaf, but the photosynthetic cells are simultaneously synthetic parenchyma, and the epidermis cells are boundary parenchyma. Notice that the lower half of the leaf has much larger intercellular spaces – is more aerenchymatic – than the upper half. This slide is unusual in that nuclei are stained green rather than the more typical red, although both colors are artificial – unstained nuclei are colorless.

Transverse section of stem of sedge (Scirpus). The large white areas are intercellular spaces which make this stem light-weight (and thus more buoyant) and permit a more rapid diffusion of oxygen downward to submerged portions of the stem. The intercellular spaces are narrow tubes separated from each other by plates of parenchyma and fiber cells which causes the stem to actually be quite strong despite being lightly constructed.

Transverse section of rush (Juncus). Like the stems of Scirpusthis one of Juncus is light-weight due to having intercellular air spaces, but in Juncus they are very large spaces, and in fact as much as half the volume of the stem is just air space, not cellular material. Notice all the debris in the intercellular spaces – those are remnants of cells destroyed as neighboring cells were pulled apart. The top of the micrograph shows vascular tissues, and the white spaces arranged in circles are xylem vessels. The red band between the outer aerenchymatous cortex and the vascular tissues is the endodermis


Transverse section of sweet flag (Acorus) rhizome. Rhizomes of Acorus are thick and grow horizontally through mud, so having a light-weight structure is not especially advantageous from a support stand point, but their aerenchymatous constructionallows oxygen diffusion down to the rhizome from the leaves (the mud of marshes is low in oxygen because abundant bacteria use it in their respiration). Whereas the stems of Scirpus and Juncus have thick plates of cells separating air canals, this stem of Acorus has plates only one parenchyma cell thick. Think of how little material is used by Acorus in the growth of these stems: most stem volume is air, and the part that is cellular consists of thin primary walls, large vacuoles (mostly just water), and only a thin film of cytosol with organelles like mitochondria, endoplasmic reticulum and nuclei. If all air and water were removed from this stem, almost nothing would remain. Notice there is no debris between the plates of cells – these intercellular spaces formed as the middle lamella broke down and neighboring cells were pulled away from each other, all cells remaining alive, none being torn apart


Transverse section of leaf of cattail (Typha). Leaves of cattail have a very light-weight construction that is easy to see by just cutting or tearing open a leaf and looking at it closely. The giant air spaces are separated by plates that are not merely thin (one cell thick), even the plates themselves are mostly air. They are composed of branched parenchyma cells like the ones shown here. The red bands (two are indicated by arrows) are cross walls separating one cell from another, and each cell has from four to six “arms.” These parenchyma cells developed from small cells that had a cubical shape when the leaf was just a tiny primordium. But as the leaf grew, neighboring cells became partially unglued from each other in certain spots, and those spots developed into intercellular space. In the spots where the cells remained glued together by their middle lamella, those areas were stretched into arms. Try to imagine exactly which walls were adjacent to which when the tissue was younger.

Transverse section of leaf of Peucephyllum (no common name). The layer of large round cells is the epidermis, a type ofboundary parenchyma. The thick, red-stained layer is the cuticle, composed of the water-proof material cutin. In this species, as in most, epidermis cells secrete cutin mostly to their outer wall, not the inner one. There is some deposition on the outer parts of the radial walls also. Because these epidermis cells have thin primary walls, they are parenchyma cells. The columnar cells in the lower half of the micrograph are palisade parenchyma cells, filled with red-stained chloroplasts. The two small cells with red contents in the center of the epidermis are the two guard cells on either side of a stomatal pore. Other than the stomatal pore, there are no intercellular spaces between epidermis cells.


Transverse section of a needle leaf of pine (Pinus). The long, cylindrical needle leaves of pine have an outer chlorenchyma made up of lobed cells (upper part of low magnification view) and a central vascular bundle (lower part of low magnification view). The two tissues are separated by a boundary parenchyma known as an endodermis. The endodermis is a single layer of cells (high magnification view) that fit against each other tightly with no intercellular spaces, and which have a waterproof Casparian strip that runs completely around each cell. Endodermis would be useless as a boundary if it had intercellular spaces.
Even though these endodermis cells have an abundance of starch grains, red-stained nuclei are visible in several. The nuclei are lumpy and irregular, not spherical – this is due to being deformed by having starch grains pressing against them.
Longitudinal section of stem of milkweed (Asclepias). The long, wide cell in the center of the micrograph (marked by arrows) is a sieve tube member, one of the types of conducting parenchyma cells of phloem. Notice that both of its end walls are slightly tilted.
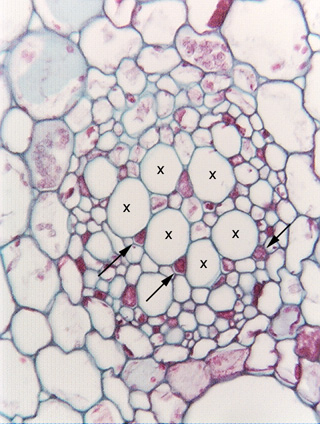
Transverse section of water lily petiole (Nymphaea). The large round, empty-looking cells marked by Xs are sieve tube members, similar to those shown in longitudinal section in Fig. 3.5-1. The small angular cells (three are indicated by arrows) that are almost filled with red contents are companion cells, which control the metabolism of the sieve tube members.